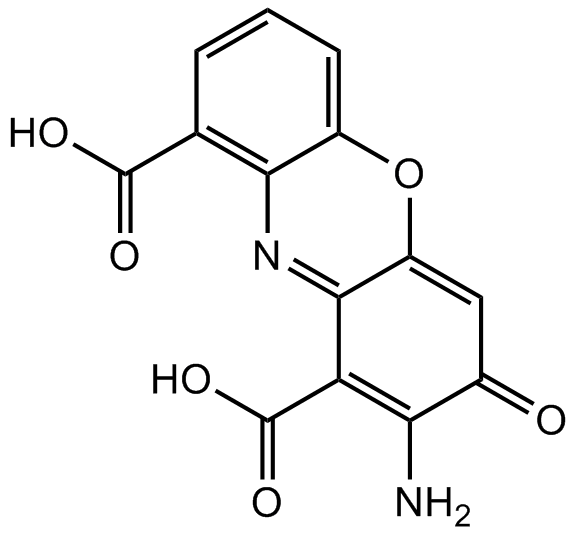
Cinnabarinic acid
An agonist of mGluR4 and active metabolite of 3-hydroxyanthranilic acid
此产品仅用于科学研究,我们不为任何个人用途提供产品和服务
-
包装价格促销价数量
-
10mg¥2275.001820.00- +
-
50mg¥10100.008080.00- +
- 货号: ajci9652
- CAS: 606-59-7
- 别名: 朱砂精酸
- 分子式: C14H8N2O6
- 分子量: 300.22
- 纯度: >98%
- 溶解度: DMSO: 25 mM (warmed)
- 储存: Store at -20°C
- 库存: 现货
Background
Cinnabarinic acid is a phenoxazinone produced by the oxidative dimerization of 3-hydroxyanthranilic acid (3-HAA) as part of the metabolism of tryptophan in the kynurenic pathway.[1],[2] It acts as a partial receptor agonist of the metabotropic glutamate receptor 4 (mGlu4), effective at 100 μM, with no activity at other mGlu receptor subtypes.[3] 3-HAA does not affect mGlu receptors, including mGlu4. Cinnabarinic acid induces apoptosis of T cells at 300-500 μM, a potency some ten times that of 3-HAA.[4]
Reference:
[1]. Subba Rao, P.V., and Vaidyanathan, C.S. Enzymic conversion of 3-hydroxyanthranilic acid into cinnabarinic acid. Partial purification and properties of rat-liver cinnabarinate synthase. Biochemistry Journal 99(2), 317-322 (1966).
[2]. Stone, T.W., Stoy, N., and Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends in Pharamacological Sciences 34(2), 136-143 (2013).
[3]. Fazio, F., Lionetto, L., Molinaro, G., et al. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Molecular Pharmacology 81(5), 643-656 (2012).
[4]. Hiramatsu, R., Hara, T., Akimoto, H., et al. Cinnabarinic acid generated from 3-hydroxyanthranilic acid strongly induces apoptosis in thymocytes through the generation of reactive oxygen species and the induction of caspase. Journal of Cellular Biochemistry 103(1), 42-53 (2008).
-

LX1606 Hippurate (Telotristat etiprate)
¥580.00 ¥725.00




























没有评价数据