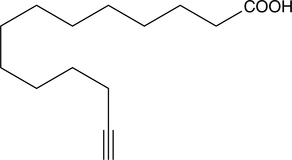
Myristic Acid Alkyne
Myristic acid containing an ω-terminal alkyne
此产品仅用于科学研究,我们不为任何个人用途提供产品和服务
-
包装价格促销价数量
-
25mg¥2337.001870.00- +
-
50mg¥3787.003030.00- +
-
100mg¥5875.004700.00- +
- 货号: ajci72758
- CAS: 82909-47-5
- 别名: 13-十四炔酸,13-alkyne Myristic Acid
- 分子式: C14H24O2
- 分子量: 224.3
- 纯度: >98%
- 溶解度: 10mg/mL in DMSO,10mg/mL in DMF, 12.5mg/mL in Ethanol
- 储存: Store at -20°C, protect from light
- 库存: 现货
Background
Myristic acid is a 14-carbon saturated (14:0) fatty acid. In vivo, it is commonly added covalently to the N-terminus of proteins in a co-translational process termed N-myristoylation. [1] The sirtuin SIRT6 removes this acyl group from myristoylated TNF-α, enhancing secretion.[2] Myristic acid alkyne is a form of this myristic acid with an ω-terminal alkyne. Such terminal alkyne groups can be used in linking reactions, known as click chemistry, characterized by high dependability and specificity of azide-alkyne bioconjugation reactions. [3][4]Click chemistry has only recently been applied to the study of lipids.[2][5]
Reference:
[1]. Farazi, T.A., Waksman, G., and Gordon, J.I. The biology and enzymology of protein N-myristoylation. The Journal of Biological Chemisty 276(43), 39501-39504 (2001).
[2]. Jiang, H., Khan, S., Wang, Y., et al. SIRT6 regulates TNF-α secretion through hydrolysis of long-chain fatty acyl lysine. Nature 496, 110-113 (2013).
[3]. Kolb, H.C., and Sharpless, K.B. The growing impact of click chemistry on drug discovery. Drug Discov. Today 8(24), 1128-1137 (2003).
[4]. Lutz, J.F., and Zarafshani, Z. Efficient construction of therapeutics, bioconjugates, biomaterials and bioactive surfaces using azide-alkyne "click" chemistry. Adv. Drug Deliv. Rev. 60(9), 958-970 (2008).
[5]. Vila, A., Tallman, K.A., Jacobs, A.T., et al. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chemical Research in Toxicology 21(2), 432-444 (2008).
-

LX1606 Hippurate (Telotristat etiprate)
¥580.00 ¥725.00




























没有评价数据