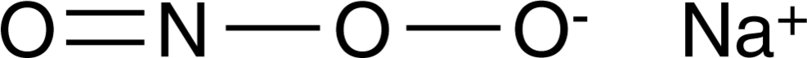
Peroxynitrite
体内NO与超氧化物反应生成过氧亚硝酸盐。
此产品仅用于科学研究,我们不为任何个人用途提供产品和服务
-
包装价格促销价数量
-
1mL¥737.00590.00- +
- 货号: ajci73446
- CAS: 14042-01-4
- 别名: 四(1-甲基-4-吡啶基)卟啉锰; Sodium Peroxynitrite
- 分子式: ONO2 ? Na
- 分子量: 85
- 纯度: >98%
- 溶解度: A solution in 0.3 M sodium hydroxide
- 储存: Storage -80°C, protect from light
- 库存: 现货
Background
Peroxynitrite is formed in vivo by the reaction of NO with superoxide.[1],[2],[3] It is a powerful oxidizing agent that can initiate lipid peroxidation, oxidize sulfhydryls, and nitrate the aromatic residues of proteins.
For long term storage, we suggest that peroxynitrite be stored as supplied at -80°C. It will be stable for at least three months.
Peroxynitrite is supplied as a solution in 0.3 M NaOH. Peroxynitrite is highly unstable and slowly decomposes even at -80°C but not to any significant extent within one month. The half-life of peroxynitrite in alkaline solutions at room temperature is about 5 hours. Peroxynitrite decomposes instantaneously under acidic conditions and the half-life at pH 7.4 is only few seconds [3]. Further dilutions of the stock solution can be performed using cold 0.3 M NaOH. We recommend that the actual concentration of peroxynitrite be measured following the procedure given below before using it in any experiments:
Thaw the peroxynitrite solution carefully and keep it on ice. Dilute an aliquot of the stock solution 40-fold with cold 0.3 M NaOH (e.g. add 25 μl of the stock to 975 μl of 0.3 M NaOH) and measure the absorbance at 302 nm with 0.3 M NaOH as blank. Concentration of the stock solution can be calculated using the extinction coefficient for peroxynitrite(1670 M-1cm-1).
体内NO与超氧化物反应生成过氧亚硝酸盐。[1] ,[2],[3]它是一种强大的氧化剂,可以引发脂质过氧化,氧化巯基,并硝酸蛋白质的芳香残基。
对于长期储存,我们建议过氧亚硝酸盐应在-80°C下储存。它将稳定至少三个月。
过氧亚硝酸盐以0.3 M NaOH溶液的形式提供。过氧亚硝酸盐高度不稳定,即使在-80°C下也会缓慢分解,但在一个月内不会分解到任何显著程度。过氧亚硝酸盐在碱性溶液中室温下的半衰期约为5小时。过亚硝酸根在酸性条件下瞬间分解,在pH 7.4下的半衰期只有几秒钟[3]。储备溶液的进一步稀释可以使用冷的0.3M NaOH进行。我们建议在任何实验中使用过氧亚硝酸盐之前,按照以下程序测量过氧亚硝酸酯的实际浓度:
小心地解冻过氧亚硝酸盐溶液,并将其放在冰上。用冷的0.3 M NaOH将储备溶液的等分试样稀释40倍(例如,将25μl储备溶液添加到975μl 0.3 M NaOH中),并用0.3 M NaOH作为空白在302 nm处测量吸光度。可以使用过氧亚硝酸盐的消光系数(1670 M-1cm-1)来计算储备溶液的浓度。
Reference:
[1]. Pryor, W.A., and Squadrito, G.L. The chemistry of peroxynitrite: A product from the reaction of nitric oxide with superoxide. American Journal of Physiology 268, L699-L722 (1995).
[2]. Beckman, J.S., and Koppenol, W.H. Nitric oxide, superoxide, and peroxynitrite: The good, the bad, and the ugly. Am. J. Physiol. 271(5 Pt 1), C1424-C1437 (1996).
[3]. Koppenol, W.H., Moreno, J.J., Pryor, W.A., et al. Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chemical Research in Toxicology 5, 834-842 (1992).
-

LX1606 Hippurate (Telotristat etiprate)
¥580.00 ¥725.00




























没有评价数据